Calciums Phosphate, Carbonate and Chloride and the World of Calcium

Calcium is important and everywhere: shells, bones, cells, brains, nerves, the heart, blood and many organs and tissues and it is hard to imagine life without calcium.
Diagram/sketch of calcium phosphate or tricalcium phosphate. Credit: Wikipedia, Edgar181
Calcium Element and Compound Properties and Uses
Calcium is an element, one of 92 basic and natural kinds of matter. Calcium is a metal (metallic element) of moderate atomic weight of 40. Calcium has 20 protons and 20 neutrons in the nucleus and 20 electrons. Each electron bears a charge of minus 1 as they circle rapidly in the electron shells or orbits and these electron negative charges balance the 1+ charge of each proton in the calcium nucleus. When the outermost orbit loses two electrons calcium becomes ionized and unbalanced in charge and becomes positively charged because there are only 18 electrons and 20 protons ( 20proto18 electrons = a 2+ charge). Ionization in chemistry means the formation of a charged atom (or an atom with a charge). This ionization occurs whenever electrons are lost or gained by an atom. The calcium atom, therefore, has a typical charge of 2+ when it is ionized and calcium ions react well with non-metallic, negatively-charged ions such as chloride (Cl-), bromide (Br-) and fluoride (F-) and charged groups such phosphate (PO4---) and carbonate (CO3--). Therefore, when calcium ions (Ca++) react with these negative ions or groups we get: calcium chloride (CaCl2), calcium phosphate or tricalcium phosphate Ca3(PO4)2 (see diagram above) and calcium carbonate CaCO3. Notice that after the chemical reactions of these charged ions are completed each of the formed compounds has no overall charge (i.e., it is uncharged). Therefore, opposite charges attract (negative attracted to positive charges and vice versa) as a chemical principle in ionic chemistry. We also see neutraliztion of all the ionic charges when the ionic bonding is completed.
Biological Significance of Calcium for Cells, Tissues, Organs, Organisms
Calcium is involved in blood clotting, the channels of all cell membranes, plant cellular walls, the beat and rhythm of the heart, the CaCO3 of stalagtites and stalagmites in caves and the shells of mollusks. To stabilize certain heart rhythms or high blood pressure calcium blocker biochemicals are used to decrease calcium entrance into cells and change the abnormal activity of the cells and tissues back to a more normal condition.
Bones are chemically calcium phosphate in structure, whereas shells are calcium carbonate. The phosphate calcium is light and stronger and more moldable than the heavier and more brittle calcium carbonate of shells.
Osteoporesis is an important degenerative bone disease of older people in general which is known by the decrease in amount and density of calcium in the bones. When this happens the bones become brittle and fragile and tend to break much easier. Osteoporesis is related to changes in the hormones and chemistry of the body. The use of vitamin D3 and CaCl2 (calcium chloride) supplements such as Citracal may be very beneficial for the prevention and reversal of this bone disease of aging. A medical doctor consultation is very important and yearly health exams are recommended for all regardless of age.
The next time you see a snail, clam, oyster or mussel remember the shell is made of calcium carbonate. And if that snail is in a cave with stalagtites and stalagmites both the cave compounds and the shell would be the same chemically.
Whenever you see a bone of a chicken, dinosaur or any other animal remember that it is calcium phosphate. The shell is an animal's exoskeleton and the bone is part of an animal's endoskeleton. Animals with exoskeletons of calcium carbonate do not have bones (endoskeltons) of calcium phosphate. And animals with endoskeltons made of bone (calcium phosphate) do not have exoskeletons of calcium carbonate. This was repeated for emphasis and so that you can remember it forever. Think about that and remember "The Sciencesuperschool.com Rule of Exo- and Endo- Skeletons which says: a skeleton on the inside means no skeleton on the outside and a skeleton outside means no skeleton inside." A final rule to remember is that some animals and creatures have no skeleton at all: bacteria, amebae and other protozooans, jellyfish are some examples. Plants lack skeletons bacause they use the fibers of cellulose or wood to fashion their lives and make their structures of trunks, stems branches, leaves, spores, cones and flowers.
If someone you know is a doctor's patient they may be using a calcium-blocker medication (drug) to block or slow down the entrance of calcium ions into the cells and change some abnormal cell functions to make things better such as blood pressure or heart beats.
Diagram/sketch of calcium phosphate or tricalcium phosphate. Credit: Wikipedia, Edgar181
Calcium Element and Compound Properties and Uses
Calcium is an element, one of 92 basic and natural kinds of matter. Calcium is a metal (metallic element) of moderate atomic weight of 40. Calcium has 20 protons and 20 neutrons in the nucleus and 20 electrons. Each electron bears a charge of minus 1 as they circle rapidly in the electron shells or orbits and these electron negative charges balance the 1+ charge of each proton in the calcium nucleus. When the outermost orbit loses two electrons calcium becomes ionized and unbalanced in charge and becomes positively charged because there are only 18 electrons and 20 protons ( 20proto18 electrons = a 2+ charge). Ionization in chemistry means the formation of a charged atom (or an atom with a charge). This ionization occurs whenever electrons are lost or gained by an atom. The calcium atom, therefore, has a typical charge of 2+ when it is ionized and calcium ions react well with non-metallic, negatively-charged ions such as chloride (Cl-), bromide (Br-) and fluoride (F-) and charged groups such phosphate (PO4---) and carbonate (CO3--). Therefore, when calcium ions (Ca++) react with these negative ions or groups we get: calcium chloride (CaCl2), calcium phosphate or tricalcium phosphate Ca3(PO4)2 (see diagram above) and calcium carbonate CaCO3. Notice that after the chemical reactions of these charged ions are completed each of the formed compounds has no overall charge (i.e., it is uncharged). Therefore, opposite charges attract (negative attracted to positive charges and vice versa) as a chemical principle in ionic chemistry. We also see neutraliztion of all the ionic charges when the ionic bonding is completed.
Biological Significance of Calcium for Cells, Tissues, Organs, Organisms
Calcium is involved in blood clotting, the channels of all cell membranes, plant cellular walls, the beat and rhythm of the heart, the CaCO3 of stalagtites and stalagmites in caves and the shells of mollusks. To stabilize certain heart rhythms or high blood pressure calcium blocker biochemicals are used to decrease calcium entrance into cells and change the abnormal activity of the cells and tissues back to a more normal condition.
Bones are chemically calcium phosphate in structure, whereas shells are calcium carbonate. The phosphate calcium is light and stronger and more moldable than the heavier and more brittle calcium carbonate of shells.
Osteoporesis is an important degenerative bone disease of older people in general which is known by the decrease in amount and density of calcium in the bones. When this happens the bones become brittle and fragile and tend to break much easier. Osteoporesis is related to changes in the hormones and chemistry of the body. The use of vitamin D3 and CaCl2 (calcium chloride) supplements such as Citracal may be very beneficial for the prevention and reversal of this bone disease of aging. A medical doctor consultation is very important and yearly health exams are recommended for all regardless of age.
The next time you see a snail, clam, oyster or mussel remember the shell is made of calcium carbonate. And if that snail is in a cave with stalagtites and stalagmites both the cave compounds and the shell would be the same chemically.
Whenever you see a bone of a chicken, dinosaur or any other animal remember that it is calcium phosphate. The shell is an animal's exoskeleton and the bone is part of an animal's endoskeleton. Animals with exoskeletons of calcium carbonate do not have bones (endoskeltons) of calcium phosphate. And animals with endoskeltons made of bone (calcium phosphate) do not have exoskeletons of calcium carbonate. This was repeated for emphasis and so that you can remember it forever. Think about that and remember "The Sciencesuperschool.com Rule of Exo- and Endo- Skeletons which says: a skeleton on the inside means no skeleton on the outside and a skeleton outside means no skeleton inside." A final rule to remember is that some animals and creatures have no skeleton at all: bacteria, amebae and other protozooans, jellyfish are some examples. Plants lack skeletons bacause they use the fibers of cellulose or wood to fashion their lives and make their structures of trunks, stems branches, leaves, spores, cones and flowers.
If someone you know is a doctor's patient they may be using a calcium-blocker medication (drug) to block or slow down the entrance of calcium ions into the cells and change some abnormal cell functions to make things better such as blood pressure or heart beats.
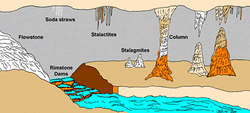
Calcium Carbonate: Caves, Speleothems, Stalagtites, Stalagmites, Calcite & Aragonite Crystals

Calcium carbonate "straw" forms and aragonite (spidery, crystal fingers).
The world of calcium carbonate and calcium carbonate crystals is a wonder to behold with its array of calcite and aragonite crytsal forms and the speleothems of stalagmites and stalagtites.
Calcium Carbonate Terms and Definitions to Know
Before we discuss the formation of limestone caves and aspects of the chemistry of caves and crystyals here are definitions to understand:
Calcium Carbonate - a common chemical compound formed by the ionic bonding of calcium and carbonate.
Carbon Dioxide - a gas present in the air at 0.3 % concentration. This gas can dissolve in water (H20) to form carbonic acid (H2CO3), a weak acid.
Aragonite - a common crystal type or form of calcium carbonate.
Calcite - the most common and stable crystal form of calcium carbonate.
Limestone - calcium carbonate rock or stone.
Speleothems - cave formations or structures deposited as calcium carbonate straws, stalagmites, stalagtites, and other crystalline forms such as mounds and rivers.
Columns - calcium carbonate formations that extend from the floor to the ceiling of a cave.
Stalagmites - calcium carbonate formations in mounds on the ground which point upward. A way to remember stalagmite is the "g" reminds us of the "ground" and "mighty" is an image of the ground's mighty fingers or arms rising upward to support the cave ceiling.
Stalagtites - calcium carbonate formations that hang from the ceiling. A way to remember the term is "top" of the cave with "tights" (longjohns or ski pants) hanging downward from above.
Caves, Limestone, Carbonate and Speleothems
Speleothems are related to three main concepts: water, calcium carbonate and acid. Rainwater moves through organic matter in the soil above the limestone rock and carbon dioxide gas in the soil reacts with water to form carbonic acid. Next, this weak acid (carbonic acid) flows and creeps through crevices, fissures and cracks in the limestone. This acid causes some calcium carbonate (limestone) to slowly dissolve in the acid solution. Rapidly moving and large amounts of water erode limestone and help to form natural caves – natural water excavations (erosions, cavities) of limestone. When acidic water with calcium and carbonate is exposed to reduced pressure and cave air, the carbon dioxide gas escapes. This event is similar to uncapping carbonated soda or champagne. When this happens the pressure inside the container decreases as the CO2 fizzes and bubbles upward and outward. The CO2 gas escapes from the water and this causes the water to become less acid and more alkaline, i.e. the pH rises. This depressurization and pH change causes calcite to be redeposited as the calcium carbonate reforms on cave walls, ceilings (stalagtites and straws) and floors (stalagmites and mounds).
Notice that as the calcium carbonate seeps, flows, drips and drops, the calcite precipitates and condenses in various shapes, forms and configuarations. The cave ceiling displays straws and hanging stalagtites which get larger over time. Excess calcium carbonate water drops onto the cave floor and builds as mounds and stalagmites.
Speleology is the study of caves.
Calcium Carbonate Terms and Definitions to Know
Before we discuss the formation of limestone caves and aspects of the chemistry of caves and crystyals here are definitions to understand:
Calcium Carbonate - a common chemical compound formed by the ionic bonding of calcium and carbonate.
Carbon Dioxide - a gas present in the air at 0.3 % concentration. This gas can dissolve in water (H20) to form carbonic acid (H2CO3), a weak acid.
Aragonite - a common crystal type or form of calcium carbonate.
Calcite - the most common and stable crystal form of calcium carbonate.
Limestone - calcium carbonate rock or stone.
Speleothems - cave formations or structures deposited as calcium carbonate straws, stalagmites, stalagtites, and other crystalline forms such as mounds and rivers.
Columns - calcium carbonate formations that extend from the floor to the ceiling of a cave.
Stalagmites - calcium carbonate formations in mounds on the ground which point upward. A way to remember stalagmite is the "g" reminds us of the "ground" and "mighty" is an image of the ground's mighty fingers or arms rising upward to support the cave ceiling.
Stalagtites - calcium carbonate formations that hang from the ceiling. A way to remember the term is "top" of the cave with "tights" (longjohns or ski pants) hanging downward from above.
Caves, Limestone, Carbonate and Speleothems
Speleothems are related to three main concepts: water, calcium carbonate and acid. Rainwater moves through organic matter in the soil above the limestone rock and carbon dioxide gas in the soil reacts with water to form carbonic acid. Next, this weak acid (carbonic acid) flows and creeps through crevices, fissures and cracks in the limestone. This acid causes some calcium carbonate (limestone) to slowly dissolve in the acid solution. Rapidly moving and large amounts of water erode limestone and help to form natural caves – natural water excavations (erosions, cavities) of limestone. When acidic water with calcium and carbonate is exposed to reduced pressure and cave air, the carbon dioxide gas escapes. This event is similar to uncapping carbonated soda or champagne. When this happens the pressure inside the container decreases as the CO2 fizzes and bubbles upward and outward. The CO2 gas escapes from the water and this causes the water to become less acid and more alkaline, i.e. the pH rises. This depressurization and pH change causes calcite to be redeposited as the calcium carbonate reforms on cave walls, ceilings (stalagtites and straws) and floors (stalagmites and mounds).
Notice that as the calcium carbonate seeps, flows, drips and drops, the calcite precipitates and condenses in various shapes, forms and configuarations. The cave ceiling displays straws and hanging stalagtites which get larger over time. Excess calcium carbonate water drops onto the cave floor and builds as mounds and stalagmites.
Speleology is the study of caves.
Calcium Channels, Proteins, Enzymes, Actin & Signaling in the Brain
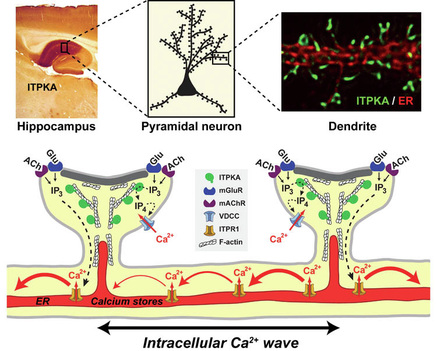
The brain of higher animals is complex in structure and function. Scientists study how the brain receives and stores information such as facts and events and can later recall this information.
The ability to recall brain-stored information is important to the function and survival of all higher organisms. Dr. Michael J. Schell is Assistant Prof. at the Uniformed Services University of the Health Sciences at Johns Hopkins. He studies animal brains and how brain signals and memories are related. Calcium is a role player in these studies and Dr Schell notes that:
1, the brain records sensory experiences in under a second, yet memories may last a lifetime – this is a big mystery.
2. Pyramidal neuron dendrites (see diagram here) have surface dendritic spines, which receive signals from other neurons.
3. The dendritic spines rapidly modify during learning (your brain dendritic spines are doing that now).
Diagram of the Structure and Function of Dendrites, Proteins and Ions of the Rat Brain.
Credit: Dr. Schell of the Uniformed Services University of the Health Sciences
4. Dendritic spines isolate calcium signals of high dynamic range inside microdomains and somehow integrate synaptic input of milliseconds to seconds.
5. Over longer timescales, the connectivity, shape, and number of spines change and possibly are involved in long-term memory storage.
Dr Schell and co-investigators study how calcium signals influence spine structure via the actin cytoskeleton (a protein network inside cells). The hippocampal pyramidal neurons are studied by live cell imaging. The actin cytoskeleton has spines and a rich amount of the enzyme inositol trisphosphate 3-kinase A (ITPKA, shown in red the diagram here). ITPKA binds next to a dynamic pool of actin filaments. ITPKA itself influences calcium signals because as it phosphorylates (adds phosphates) the second messenger inositol trisphosphate (IP3) simultaneously turns off the signal to release calcium from intracellular stores.
Dr Schell's research on synaptic calcium signal pathways may reveal the protein changes which regulate the actin microstructure in spines. Finally, he wants to find and reveal those lasting molecular-structural changes at the synapse that lead to learning and memory. Now, that would be terrific. Think about this outline of events:
calcium signals--->protein and enzyme changes--->actin regulation and final molecular-structural changes. Looks simple when you outline it like that don't you think?
If thinking about all this makes you a little tired, then you now know that all good brain work always requires a lot of chemical energy (as ATP, GTP and ITP for example) and this brain work causes important cell changes to occur. That's O.K. and that's worthwhile.
Have fun while learning and remember that calcium is important to learning, bones, shells and a host of other chemical and biological things that we sometimes take for granted.
The ability to recall brain-stored information is important to the function and survival of all higher organisms. Dr. Michael J. Schell is Assistant Prof. at the Uniformed Services University of the Health Sciences at Johns Hopkins. He studies animal brains and how brain signals and memories are related. Calcium is a role player in these studies and Dr Schell notes that:
1, the brain records sensory experiences in under a second, yet memories may last a lifetime – this is a big mystery.
2. Pyramidal neuron dendrites (see diagram here) have surface dendritic spines, which receive signals from other neurons.
3. The dendritic spines rapidly modify during learning (your brain dendritic spines are doing that now).
Diagram of the Structure and Function of Dendrites, Proteins and Ions of the Rat Brain.
Credit: Dr. Schell of the Uniformed Services University of the Health Sciences
4. Dendritic spines isolate calcium signals of high dynamic range inside microdomains and somehow integrate synaptic input of milliseconds to seconds.
5. Over longer timescales, the connectivity, shape, and number of spines change and possibly are involved in long-term memory storage.
Dr Schell and co-investigators study how calcium signals influence spine structure via the actin cytoskeleton (a protein network inside cells). The hippocampal pyramidal neurons are studied by live cell imaging. The actin cytoskeleton has spines and a rich amount of the enzyme inositol trisphosphate 3-kinase A (ITPKA, shown in red the diagram here). ITPKA binds next to a dynamic pool of actin filaments. ITPKA itself influences calcium signals because as it phosphorylates (adds phosphates) the second messenger inositol trisphosphate (IP3) simultaneously turns off the signal to release calcium from intracellular stores.
Dr Schell's research on synaptic calcium signal pathways may reveal the protein changes which regulate the actin microstructure in spines. Finally, he wants to find and reveal those lasting molecular-structural changes at the synapse that lead to learning and memory. Now, that would be terrific. Think about this outline of events:
calcium signals--->protein and enzyme changes--->actin regulation and final molecular-structural changes. Looks simple when you outline it like that don't you think?
If thinking about all this makes you a little tired, then you now know that all good brain work always requires a lot of chemical energy (as ATP, GTP and ITP for example) and this brain work causes important cell changes to occur. That's O.K. and that's worthwhile.
Have fun while learning and remember that calcium is important to learning, bones, shells and a host of other chemical and biological things that we sometimes take for granted.