Chemistry – Protons, Neutrons, Electrons, The Atom; Elements, 92 Natural Types; Atomic Bond Types
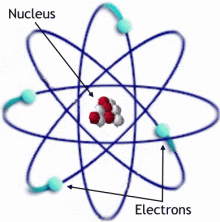
Atom, the Smallest Part of an Element
Chemistry is the Science of Matter as Atoms, Elements, and Compounds
Chemistry is the study of the simplest atom and element hydrogen to the largest biochemicals known — proteins and the marvelous code of life — DNA. Chemistry is the study of molecules and compounds that are fashioned from the basic elements of matter here on earth.
Atoms, Elements, Compounds, and Chemical Bonds
The atom, is the smallest part of an element that still has the properties of that element.
What is an element? An element is one of the 92 basic types of matter (anything that occupies space and has mass). Each element is distinct and has properties. Properties of each element are unique and special. Gold, silver, iron, carbon, oxygen, nitrogen, hydrogen, phosphorous, and calcium are just 9 elements among the amazing 92 natural elements. Each element has a unique mass that relates to the heavy nucleus (containing protons and neutrons – see photo of the atom). Each element also has unique chemical characteristics. Hydrogen, the simplest of all the elements has 1 electron and 1 proton. Other atoms of elements have neutrons, and these neutrons add to the element's weight, and make each element distinctive. Two elements discussed in detail on this site are calcium and hydrogen for those interested in learning more about elements.
Atoms of elements can combine, interact, and become chemically bonded to one another to form distinct compounds which each have unique properties. Examples of some simple compounds are water (H2O), carbon dioxide (CO2), carbon monoxide (CO), methane (CH4), and ammonia (NH3). Water as a compound is revealed as one of the most important compounds on our planet – life without water seems almost impossible to imagine or believe.
Chemistry is a wonderful and fascinating science —it is the very substance of everything alive or inanimate.
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.
Chemistry is the study of the simplest atom and element hydrogen to the largest biochemicals known — proteins and the marvelous code of life — DNA. Chemistry is the study of molecules and compounds that are fashioned from the basic elements of matter here on earth.
Atoms, Elements, Compounds, and Chemical Bonds
The atom, is the smallest part of an element that still has the properties of that element.
What is an element? An element is one of the 92 basic types of matter (anything that occupies space and has mass). Each element is distinct and has properties. Properties of each element are unique and special. Gold, silver, iron, carbon, oxygen, nitrogen, hydrogen, phosphorous, and calcium are just 9 elements among the amazing 92 natural elements. Each element has a unique mass that relates to the heavy nucleus (containing protons and neutrons – see photo of the atom). Each element also has unique chemical characteristics. Hydrogen, the simplest of all the elements has 1 electron and 1 proton. Other atoms of elements have neutrons, and these neutrons add to the element's weight, and make each element distinctive. Two elements discussed in detail on this site are calcium and hydrogen for those interested in learning more about elements.
Atoms of elements can combine, interact, and become chemically bonded to one another to form distinct compounds which each have unique properties. Examples of some simple compounds are water (H2O), carbon dioxide (CO2), carbon monoxide (CO), methane (CH4), and ammonia (NH3). Water as a compound is revealed as one of the most important compounds on our planet – life without water seems almost impossible to imagine or believe.
Chemistry is a wonderful and fascinating science —it is the very substance of everything alive or inanimate.
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.