Pure Water Facts, Properties – Chemistry and Biology
Water, Dihydrogen Oxide, 2 Hydrogen Atoms, 1 Oxygen
Life and water are bound together in the wonderful sciences of biology and chemistry. Water molecules are unique compounds. Water is formed by the bonding of 2 hydrogen atoms with 1 oxygen atom to yield H2O. The name di-hydrogen mon-oxide signifies the 2 hydrogens to 1 oxygen bonding and ratio of atoms. Learn more about this most important inorganic compound that accounts for about 65% of anyone's body weight.
Pure Water, Covalent and Hydrogen Bonds, Bipolar Attractions
Water always has 2 hydrogen atoms linked to 1 oxygen atom. Atoms that share electrons can form covalent bonds with other atoms. For now, simply remember that a covalent is the strongest chemical bond and is a bond of shared electrons. To imagine covalent bonding shake hands with someone else and hold on tightly. Or, simply clasp or grasp one's own left hand and right hand together. The fingers of each hand are like electrons, and each finger still belongs to the hand to which it is attached. The clasping fingers represent shared electrons between the 2 atoms.
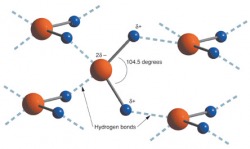
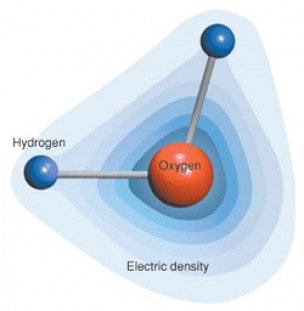
Water molecules are bipolar, or dipolar (see photo below. This means that hydrogen atoms tend to have an overall positive charge distribution in the water molecule, and the oxygen tends to be negative due to the high concentration of electrons around the oxygen atom. Another way of saying this is that electrons tend to move or distribute strongly with oxygen and give it a negative charge. In contrast, hydrogen has less electron density than oxygen, and the protons in the hydrogen nucleus give it a overall positive charge. Imagine a a dozen or more bar magnets with north and south poles. Magnets with opposite poles attract (N and S), similar poles repel (S to S, N to N).
In summary, water molecules act like little magnets — opposite charges attract, and like charges repel one another. Bipolarity creates some very special and unique features of water. These are the special properties of water. These properties relate to biplority of water and the phenomenon of hydrogen bonding. The protons of the hydrogen nucleus are attracted to the electronegative portions of other atoms like oxygen. H-bonding is very common in water, and within large molecules such as proteins and DNA. Dipolarity is shown in the photo below.
Pure Water, Wondrous Properties
The facts and properties of water are:
Life without water is hard to imagine, becuase there is no other known solvent that has water's properties. Compounds may dissolve or suspend in water, and the solutions or suspensions of molecules permit them to diffuse and distribute freely in the water matrix. Chemical and biochemical interactions are promoted in water environments. When water is evaporated as in drying , or when water freezes, movement and free associations of compounds and molecules are limited or do not happen at all. Water is considered such an excellent solvent because so many different compounds dissolve in water and form water solutions. There is no other known solvent compound that has the truly unique properties of water. In fact, it is hard to imagine that if extra-terrestrial life exists, that water would be essential for it, just as it is on planet earth. In summary, molecular movements and interactions, and life itself, all are promoted by water.
Pure Water, Universal Solvent for Life and Good Health
Pure water is important for overall health of cells, tissues and the entire body. Potable (drinkable) water should be free of any dangerous compounds, elements (e.g., lead, mercury, arsenic) or toxins (e.g., dioxins, DDT, herbicides, pesticides).
Protection of water from contamination, and maintenance of clean sources of water, promotes healthy people, plants and animals. Wherever water is found in streams, rivers, lakes, underground waters, earth's oceans and seas aquatic and terrestrial life depends on pure sources of this vital resource.
Pollutants degrade the quality of the water, and can harm creatures which always need and consume water. Microorganisms, lower plants, and animals, in combination with other factors such as natural and artificial aeration and filtration, contribute to help purify water for human drinking, cooking and other important purposes. Further, specialized filtration systems and mechanisms also can be used to restore water to a more pure potable state.
Humans must be be good stewards of the planet. Human pollution of water and air causes immediate and potential, long-term damage. Whenever water is used it is a reminder to all that water sources and resources are critical to daily life, and our longterm survival.
Resource
Alters, S and B. Alters. 2006, Biology. John Wiley & Sons Inc., Hoboken, N.J. 755 pp
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.
Pure Water, Covalent and Hydrogen Bonds, Bipolar Attractions
Water always has 2 hydrogen atoms linked to 1 oxygen atom. Atoms that share electrons can form covalent bonds with other atoms. For now, simply remember that a covalent is the strongest chemical bond and is a bond of shared electrons. To imagine covalent bonding shake hands with someone else and hold on tightly. Or, simply clasp or grasp one's own left hand and right hand together. The fingers of each hand are like electrons, and each finger still belongs to the hand to which it is attached. The clasping fingers represent shared electrons between the 2 atoms.
Water molecules are bipolar, or dipolar (see photo below. This means that hydrogen atoms tend to have an overall positive charge distribution in the water molecule, and the oxygen tends to be negative due to the high concentration of electrons around the oxygen atom. Another way of saying this is that electrons tend to move or distribute strongly with oxygen and give it a negative charge. In contrast, hydrogen has less electron density than oxygen, and the protons in the hydrogen nucleus give it a overall positive charge. Imagine a a dozen or more bar magnets with north and south poles. Magnets with opposite poles attract (N and S), similar poles repel (S to S, N to N).
In summary, water molecules act like little magnets — opposite charges attract, and like charges repel one another. Bipolarity creates some very special and unique features of water. These are the special properties of water. These properties relate to biplority of water and the phenomenon of hydrogen bonding. The protons of the hydrogen nucleus are attracted to the electronegative portions of other atoms like oxygen. H-bonding is very common in water, and within large molecules such as proteins and DNA. Dipolarity is shown in the photo below.
Pure Water, Wondrous Properties
The facts and properties of water are:
- excellent solvent, water dissolves many compunds to form solutions; larger molecules do not dissolve, but remain suspended. Examples: many salts, sugars, amino acids dissolve, and the larger molecules form colloids that float and are suspended in the water (examples, milk and gelatin proteins.
- highest density of water occurs at 4 degrees Centigrade; molecules sink downward as they cool.
- lowest density at O degrees C; water changes from liquid to solid (phase change) and forms ice crystals that float.
- high surface tension, enables objects to float on the surface (examples, needle, water strider insects and other bugs).
- capillarity; water molecules have dipolarity, attarct and pull one another along narrow channels and surfaces.
- high specific heat and heat of vaporization; 1 calorie is needed to raise 1 gram of water, 1 degree Centigrade; 540 calories needed to cause 1 gram of water to change from a liquid water to steam vapor. Water retain heat well, and upon evaporation, water carries heat away with it. This means that sweating or panting has a cooling effect and this cooling protects animals from damage due to body heat.
- small size of water molecules and the kinetic energy of water molecules contribute to the Brownian motion of larger molecules and particles. Brownian motion is the erratic, irregular and random movement of objects suspended in water.
Life without water is hard to imagine, becuase there is no other known solvent that has water's properties. Compounds may dissolve or suspend in water, and the solutions or suspensions of molecules permit them to diffuse and distribute freely in the water matrix. Chemical and biochemical interactions are promoted in water environments. When water is evaporated as in drying , or when water freezes, movement and free associations of compounds and molecules are limited or do not happen at all. Water is considered such an excellent solvent because so many different compounds dissolve in water and form water solutions. There is no other known solvent compound that has the truly unique properties of water. In fact, it is hard to imagine that if extra-terrestrial life exists, that water would be essential for it, just as it is on planet earth. In summary, molecular movements and interactions, and life itself, all are promoted by water.
Pure Water, Universal Solvent for Life and Good Health
Pure water is important for overall health of cells, tissues and the entire body. Potable (drinkable) water should be free of any dangerous compounds, elements (e.g., lead, mercury, arsenic) or toxins (e.g., dioxins, DDT, herbicides, pesticides).
Protection of water from contamination, and maintenance of clean sources of water, promotes healthy people, plants and animals. Wherever water is found in streams, rivers, lakes, underground waters, earth's oceans and seas aquatic and terrestrial life depends on pure sources of this vital resource.
Pollutants degrade the quality of the water, and can harm creatures which always need and consume water. Microorganisms, lower plants, and animals, in combination with other factors such as natural and artificial aeration and filtration, contribute to help purify water for human drinking, cooking and other important purposes. Further, specialized filtration systems and mechanisms also can be used to restore water to a more pure potable state.
Humans must be be good stewards of the planet. Human pollution of water and air causes immediate and potential, long-term damage. Whenever water is used it is a reminder to all that water sources and resources are critical to daily life, and our longterm survival.
Resource
Alters, S and B. Alters. 2006, Biology. John Wiley & Sons Inc., Hoboken, N.J. 755 pp
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.