pH, Hydrogen Ions (H+) – A Critical Factor for Life and Chemistry
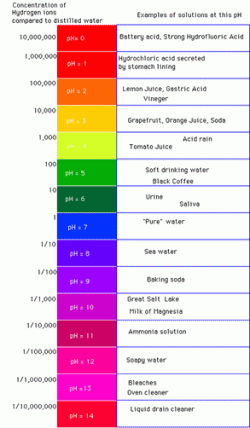
pH Numerical Range Extends from O to 14
pH, Proton or Hydrogen Ion Concentration Rules
Much in the world of biology and biochemistry is strongly influenced by pH. pH values are known and determined both inside and outside the body, and solutions and mixtures alike are frequently checked. pH is measured for water, soils, blood, and other clinical specimens obtained from patients. pH is an important value that has significance and influences and effects. If the pH is not within a certain range, some reactions just do not occur. Spas, hot tubs and swimming pools are checked for pH to assure that chlorine or bromine disinfectants will work effectively. In summary, just about everything in the chemical and biological world is ruled and influenced by pH — see why and how this happens.
pH, Ion Formation by Water, Fact Check
If a sample of absolutely pure water is obtained, with nothing dissolved or contained it, a pH value of 7.0 is registered. The entire pH scale runs from the most acid value of 0, to the most basic value of 14. A pH of 7.0 is considered neutral pH, and it is neither acid or basic. Any value below 7 is an acid value, and any value above 7 is a basic value. The most acid and the most basic values are 0 and 14, respectively.
Pure water is known to dissociate or ionzize normally. Approximately, 1 molecule of the water compound ionizes out of every 10 million (10 to the 7th power, or 1/10,000,000). When pure water ionizes dissociates or separates to form 1 H + (proton) and 1 (OH) - hydroxyl ions. Therefore, if the formula for water is written as H2O or HOH (water molecule) —> H+ and (OH)-. When pure water ionizes equal amounts of protons and hydroxyl ions form.
pH Details and Definition – Logarithms, Reciprocal Logs
pH is the logarithm (to the base 10) of the reciprocal (inverted number) of the hydrogen ion concentration. If 1 in 100 million is reciprocated the value is 100 million, and the log 10 value of 100 million is 8.0.
pH is always a measure of hydrogen ion concentration. Thus, pH values of 5.0, 4.0, 3.0, 2.0 and 1.0 express, respectively, that 1 in 100,000, 1 in 10,000, 1/1,000, 1/100 and 1 in 10 concentrations of hydrogen ions (protons) are present. pH values of 3 to 6 indicate mild to weak acidities typical of weaker, organic acids (such as citric, acetic and lactic acids). The strong, inorganic acids (HCl, hydrochloric acid; HNO3, nitric acid; H2SO4, sulfuric acid) give values of 1, 2 or even 0. Strong bases such as NaOH (sodium hydroxide) and KOH (potassium hydroxide) have pH values that are in the range of 13 and 14. Bases have larger concentrations of (OH)-, hydroxyl ions. Both Inorganic acids and bases have ionic bonds that promote complete and and ionization and promote corresponding, low acidity or highly basic values.
Much in the world of biology and biochemistry is strongly influenced by pH. pH values are known and determined both inside and outside the body, and solutions and mixtures alike are frequently checked. pH is measured for water, soils, blood, and other clinical specimens obtained from patients. pH is an important value that has significance and influences and effects. If the pH is not within a certain range, some reactions just do not occur. Spas, hot tubs and swimming pools are checked for pH to assure that chlorine or bromine disinfectants will work effectively. In summary, just about everything in the chemical and biological world is ruled and influenced by pH — see why and how this happens.
pH, Ion Formation by Water, Fact Check
If a sample of absolutely pure water is obtained, with nothing dissolved or contained it, a pH value of 7.0 is registered. The entire pH scale runs from the most acid value of 0, to the most basic value of 14. A pH of 7.0 is considered neutral pH, and it is neither acid or basic. Any value below 7 is an acid value, and any value above 7 is a basic value. The most acid and the most basic values are 0 and 14, respectively.
Pure water is known to dissociate or ionzize normally. Approximately, 1 molecule of the water compound ionizes out of every 10 million (10 to the 7th power, or 1/10,000,000). When pure water ionizes dissociates or separates to form 1 H + (proton) and 1 (OH) - hydroxyl ions. Therefore, if the formula for water is written as H2O or HOH (water molecule) —> H+ and (OH)-. When pure water ionizes equal amounts of protons and hydroxyl ions form.
pH Details and Definition – Logarithms, Reciprocal Logs
pH is the logarithm (to the base 10) of the reciprocal (inverted number) of the hydrogen ion concentration. If 1 in 100 million is reciprocated the value is 100 million, and the log 10 value of 100 million is 8.0.
pH is always a measure of hydrogen ion concentration. Thus, pH values of 5.0, 4.0, 3.0, 2.0 and 1.0 express, respectively, that 1 in 100,000, 1 in 10,000, 1/1,000, 1/100 and 1 in 10 concentrations of hydrogen ions (protons) are present. pH values of 3 to 6 indicate mild to weak acidities typical of weaker, organic acids (such as citric, acetic and lactic acids). The strong, inorganic acids (HCl, hydrochloric acid; HNO3, nitric acid; H2SO4, sulfuric acid) give values of 1, 2 or even 0. Strong bases such as NaOH (sodium hydroxide) and KOH (potassium hydroxide) have pH values that are in the range of 13 and 14. Bases have larger concentrations of (OH)-, hydroxyl ions. Both Inorganic acids and bases have ionic bonds that promote complete and and ionization and promote corresponding, low acidity or highly basic values.
pH Measurement with Papers, Meters, Solutions

pH Paper Measurements 4 to 10
pH Determination by Color Indicators and Electronic Devices
These methods permit accurate pH measurements to be made:
1. special color indicators (incorporated into pH paper, or solutions) such as methyl violet, congo red, methyl red, phenol red, litmus and phenolphthalein. The pH of the solution influences the color by changing the structure of the indicator molecule. This pH-caused change is temporary in the molecule. The color may change back again to another color when another pH change occurs. Each indicator has a different range of effectiveness. Some indicators have narrow pH ranges, some have broader ranges.
2. pH meters can also detect hydrogen ion concentrations and differences. These electroninc meters use battery power or direct electrical line power to measure protons by means of a special electrode with KCl. In the presence of H+ (protons), the current flows and is measured. The meter is first calibrated with standardized, known pH solutions. The measuring electrode is washed with pure water to remove any previous solutions, and then the electrode is immersed in the test solution. Readings with pH meters typically can be made throughout the full range of 0 to 14 pH.
pH Importance, Biology, Chemistry, Geology
Many compounds have typical pH values associated with them as shown in the photo. Fruits and vegetables have low pH values, and battery acid is even more acidic. Pure water is always a neutral pH 7.0, and at the high end of pH (with few H+ (protons) and many OH- , hydroxyl ions) are compounds such as bleach, drain-cleaners (KOH or NaOH).
Why is pH so important? pH is significant because so many other compounds are influenced by pH. When a tooth decays, acids produced by bacteria in the mouth break down enamel and cause cavities. The acid produced by bacteria erodes the calcium phosphate of the tooth and causes enamel to wash away as Ca++ (clacium ions) and PO4--- (phosphate ions). If the decay is not remedied by dental treatment, the whole tooth eventually decays and the damage goes to the very root of the tooth and gums.
Enzymes (protein catalysts) in living systems depend upon the proper pH to work. In the stomach, pepsin enzyme works best at pH 2. In the duodenum of the small intestine, trypsin degrades proteins optimally at pH 7.5–8.0. Typically, most human enzymes perform best in a slightly alkaline environment (7.4).
Normal pH is Vital and Important to Life, Acidosis and Diabetes
Normal pH is so important because even a small shift of a few tenths from the normal pH may cause death of cells, tissues, or even the whole body. A person's body may become acidotic during episodes of uncontrolled diabetes, a disease caused by a very large amount sugar in the body. The sugar cannot get into the cells because the insulin is either lacking or defective. Because of the lack of sugar inside cells these sugar-starved cells start to use fat for energy, and fat metabolism yields glycerol and fatty acids. The fatty acids accumulate and cause the pH to drop from 7.4 to 6.8 to 7.0. The acids rapidly inactivate enzymes and cells, and unless this situation is corrected death may occur. Diabetics treat their disease by either injections of insulin or oral medications. Regular blood sugar monitoring is important for diabetics.
Animals can get diabetes too. Pet dogs, cats, horses and pigs may become diabetics, and these diabetic animals require treatment to survive. Just as medical doctors take care of humans, veterinarians are animal doctor specialists.
Diabetes as a disease of humans and other animals will be discussed later in another section.
Blood alkalosis may occur in other disease situations also, and here pH values of 7.6–7.8 or greater, are noted. Alkalosis, too, is dangerous. Typically, natural buffers in the body, such as NaHCO3 and proteins, regulate normal acid and alkaline pH changes.
Remember, pH is the hydrogen ion concentration, and normal pH is critical to life, biochemistry and many chemical reactions.
Resources
Alters, S and B. Alters. 2006, Biology. John Wiley & Sons Inc., Hoboken, N.J. 755 pp
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.
These methods permit accurate pH measurements to be made:
1. special color indicators (incorporated into pH paper, or solutions) such as methyl violet, congo red, methyl red, phenol red, litmus and phenolphthalein. The pH of the solution influences the color by changing the structure of the indicator molecule. This pH-caused change is temporary in the molecule. The color may change back again to another color when another pH change occurs. Each indicator has a different range of effectiveness. Some indicators have narrow pH ranges, some have broader ranges.
2. pH meters can also detect hydrogen ion concentrations and differences. These electroninc meters use battery power or direct electrical line power to measure protons by means of a special electrode with KCl. In the presence of H+ (protons), the current flows and is measured. The meter is first calibrated with standardized, known pH solutions. The measuring electrode is washed with pure water to remove any previous solutions, and then the electrode is immersed in the test solution. Readings with pH meters typically can be made throughout the full range of 0 to 14 pH.
pH Importance, Biology, Chemistry, Geology
Many compounds have typical pH values associated with them as shown in the photo. Fruits and vegetables have low pH values, and battery acid is even more acidic. Pure water is always a neutral pH 7.0, and at the high end of pH (with few H+ (protons) and many OH- , hydroxyl ions) are compounds such as bleach, drain-cleaners (KOH or NaOH).
Why is pH so important? pH is significant because so many other compounds are influenced by pH. When a tooth decays, acids produced by bacteria in the mouth break down enamel and cause cavities. The acid produced by bacteria erodes the calcium phosphate of the tooth and causes enamel to wash away as Ca++ (clacium ions) and PO4--- (phosphate ions). If the decay is not remedied by dental treatment, the whole tooth eventually decays and the damage goes to the very root of the tooth and gums.
Enzymes (protein catalysts) in living systems depend upon the proper pH to work. In the stomach, pepsin enzyme works best at pH 2. In the duodenum of the small intestine, trypsin degrades proteins optimally at pH 7.5–8.0. Typically, most human enzymes perform best in a slightly alkaline environment (7.4).
Normal pH is Vital and Important to Life, Acidosis and Diabetes
Normal pH is so important because even a small shift of a few tenths from the normal pH may cause death of cells, tissues, or even the whole body. A person's body may become acidotic during episodes of uncontrolled diabetes, a disease caused by a very large amount sugar in the body. The sugar cannot get into the cells because the insulin is either lacking or defective. Because of the lack of sugar inside cells these sugar-starved cells start to use fat for energy, and fat metabolism yields glycerol and fatty acids. The fatty acids accumulate and cause the pH to drop from 7.4 to 6.8 to 7.0. The acids rapidly inactivate enzymes and cells, and unless this situation is corrected death may occur. Diabetics treat their disease by either injections of insulin or oral medications. Regular blood sugar monitoring is important for diabetics.
Animals can get diabetes too. Pet dogs, cats, horses and pigs may become diabetics, and these diabetic animals require treatment to survive. Just as medical doctors take care of humans, veterinarians are animal doctor specialists.
Diabetes as a disease of humans and other animals will be discussed later in another section.
Blood alkalosis may occur in other disease situations also, and here pH values of 7.6–7.8 or greater, are noted. Alkalosis, too, is dangerous. Typically, natural buffers in the body, such as NaHCO3 and proteins, regulate normal acid and alkaline pH changes.
Remember, pH is the hydrogen ion concentration, and normal pH is critical to life, biochemistry and many chemical reactions.
Resources
Alters, S and B. Alters. 2006, Biology. John Wiley & Sons Inc., Hoboken, N.J. 755 pp
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.