Crude Environmental Oil – Potential Hazard to Life and Living Organisms

Oil, Heavy Crude Venzuelan, DNR Louisiana
Crude oil is a complex mixture of many chemicals and crude oil has complex properties. Crude oil spills and environmental oil contamination can have profound and dangerous effects on living cells and organisms.The difference between contained, underground crude oil versus environmental and contaminating crude oil is stark – it is a contrast of black and white, night and day, life and death. Crude oils in natural underground reservoirs are usually walled-in and safely enclosed. Drilling is needed to reach these places and then extraction of the oil can begin. Once released from their natural reservoirs crude oil may be harmful or deadly to plants and animals. Here are some important things to know about the chemistry and biology of crude oil.
Crude Oils —Chemistry, Features and Characteristics of Hydrocarbons
Overview of Temperature Used to Distill and Separate Crude Oil Mixtures
Crude oil is not simple. The three simple letters that spell "oil" hide deep and diverse chemical complexity. (Michel, J. 1992. Oil Behavior and Toxicity, Chapter 2. in Introduction to Coastal Habitats and Biological Resources for Spill Response response. (Office of Response and Restoration, NOAA, restoration.noaa.gov/book_shelf/678_Chapter2.pdf, accessed May 6, 2010)
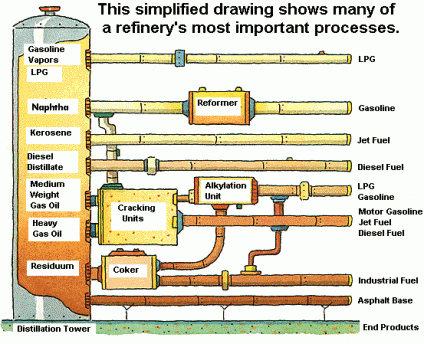
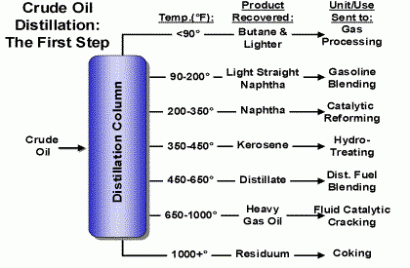
Crude oil can be taken to a refinery and processed by progressive heating and other specialized procedures. Temperature processing slowly separates or distills gases and liquids (gasoline, diesel, lubricating and heating oils) from the solids. The end result of full processing of crude oil yields useful fractions of the oil which can be collected and used as needed. The largest molecular hydrocarbon components are solids at room temperature and include tar and asphalt useful for roofs and roads.
- Crude oil is a mixture of many different hydrocarbons.
These oil hydrocarbons are composed mostly carbon and hydrogen and some oxygen and sulfur. - There are light-, medium- and heavy- weight components in crude oil.
The smallest hydrocarbons are gaseous at ordinary room temperature.
The larger hydrocarbon molecules are liquids and some are solids.
This entire crude oil complex is called a mixture of hydrocarbons. Crude oil hydrocarbons range include small-ringed benzene, toluene, xylense, kerosene, and naphthylene (which can evaporate at temperature) to larger molecules that float on the surface of the water and can become solids such as tar during evaporation or distillation.
Crude oil can be taken to a refinery and processed by progressive heating and other specialized procedures. Temperature processing slowly separates or distills gases and liquids (gasoline, diesel, lubricating and heating oils) from the solids. The end result of full processing of crude oil yields useful fractions of the oil which can be collected and used as needed. The largest molecular hydrocarbon components are solids at room temperature and include tar and asphalt useful for roofs and roads.
Crude Oil Effects on Living Cells and Living Organisms
Normal Cell Membrane Complexity is Easily Damaged by Chemicals
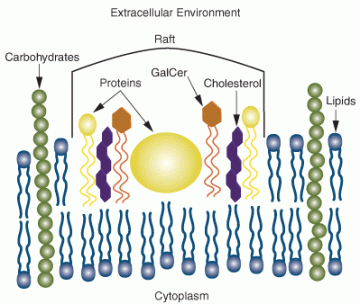
Crude oil, which directly contaminates land, sea or the air, often has toxic effects on life and living organisms of all types. Cell membranes of all living cells have large hydrophobic or an oil-like composition. The lipid or naturally-oily components of cell membranes interacts strongly with many of the toxic components of oil. Benzene, toluene, xylene, gasoline, naphthylene are just a few of the chemical compounds of crude oil that can dissolve or deform cell membranes and cause cell death. Membranes contain enzymes and transport proteins that are critical to the cell. When the cell membrane is damaged or disrupted the working membrane may not work to transport or permit passage of molecules. Furthermore, damaged cell membranes permit critical cell molecules to leak out of the cell and this contributes to cell death. Benzene-ring compounds and other crude oil chemical components are known carcinogens — agents that can cause cancer. These compounds, if they reach the nuclear DNA, may cause mutation, cell malfunctions, or even cancers that are characterized by uncontrolled cell growth and multiplication. These facts about crude oil indicate how important it is to protect living cells and organisms from crude oil and crude oil components.
Toxicity and Carcinogenicity of Volatile Crude Oil Components, Benzene Example
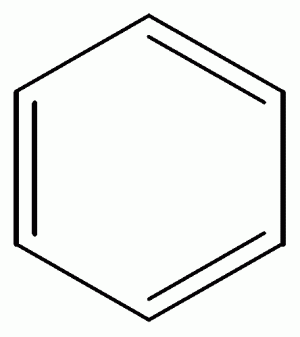
Benzene, A Simple Ringed Molecule and Carcinogen
Benzene, toluene, xylene, gasoline are among the volatile, carcinogenic components of crude oil. Benzene in a well-established toxic carcinogen. Warnings are posted at pumping stations that inform all that inhalation of gasoline fumes should be avoided to prevent inhalation of benzene, a known carcinogen. Snyder and Hedli provide a detailed overview of benzene metabolism, and they clearly indicate the great complexity associated with metabolism of this simple compound (An Overview of Benzene Metabolism, Environmental Health Perspectives, Volume 104, Supplement 6, December 1996 (ehp.niehs.nih.gov/realfiles/docs/1996/Suppl-6/snyder.html, accessed May 11, 2010)
Benzene from Oil – Cell Damage Facts:
1. bone marrow depression and leukemogenesis occurs.
2. damage to multiple types of bone marrow cells is noted.
3. several metabolites of benzene can generate toxic cell effects.
4. metabolism of benzene in the liver produces hydroxylated and ring-opened benzene products.
5. these liver metabolites are transported to the bone marrow where additional secondary metabolism occurs.
6. benzene metabolites may damage cellular macromolecules by covalent binding or oxidative damage.
7. benzene toxicity is complex.
Benzene, EPA Hazard Summary Report Sheet Summary:
"Hazard Summary-Created in April 1992; Revised in January 2000"
"Benzene is found in the air from emissions from burning coal and oil, gasoline service stations, and motor vehicle exhaust. Acute (short-term) inhalation exposure of humans to benzene may cause drowsiness, dizziness, headaches, as well as eye, skin, and respiratory tract irritation, and, at high levels, unconsciousness. Chronic (long-term) inhalation exposure has caused various disorders in the blood, including reduced numbers of red blood cells and aplastic anemia, in occupational settings. Reproductive effects have been reported for women exposed by inhalation to high levels, and adverse effects on the developing fetus have been observed in animal tests. Increased incidence of leukemia (cancer of the tissues that form white blood cells) have been observed in humans occupationally exposed to benzene. EPA has classified benzene as a Group A, human carcinogen." http://www.epa.gov/ttn/atw/hlthef/benzene.html, accessed May 11, 2010.
In summary, benzene is deceptive in its molecular simplicity. Benzene compounds readily are volatilized into the air and may be breathed in or absorbed through the skin. Benzene has great toxicity and is a human carcinogen
Benzene from Oil – Cell Damage Facts:
1. bone marrow depression and leukemogenesis occurs.
2. damage to multiple types of bone marrow cells is noted.
3. several metabolites of benzene can generate toxic cell effects.
4. metabolism of benzene in the liver produces hydroxylated and ring-opened benzene products.
5. these liver metabolites are transported to the bone marrow where additional secondary metabolism occurs.
6. benzene metabolites may damage cellular macromolecules by covalent binding or oxidative damage.
7. benzene toxicity is complex.
Benzene, EPA Hazard Summary Report Sheet Summary:
"Hazard Summary-Created in April 1992; Revised in January 2000"
"Benzene is found in the air from emissions from burning coal and oil, gasoline service stations, and motor vehicle exhaust. Acute (short-term) inhalation exposure of humans to benzene may cause drowsiness, dizziness, headaches, as well as eye, skin, and respiratory tract irritation, and, at high levels, unconsciousness. Chronic (long-term) inhalation exposure has caused various disorders in the blood, including reduced numbers of red blood cells and aplastic anemia, in occupational settings. Reproductive effects have been reported for women exposed by inhalation to high levels, and adverse effects on the developing fetus have been observed in animal tests. Increased incidence of leukemia (cancer of the tissues that form white blood cells) have been observed in humans occupationally exposed to benzene. EPA has classified benzene as a Group A, human carcinogen." http://www.epa.gov/ttn/atw/hlthef/benzene.html, accessed May 11, 2010.
In summary, benzene is deceptive in its molecular simplicity. Benzene compounds readily are volatilized into the air and may be breathed in or absorbed through the skin. Benzene has great toxicity and is a human carcinogen
Oil Spills and Seafood Safety (Fish, Shrimp, Crabs, etc.) Post Spill
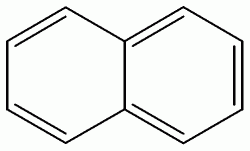
Naphthalene Molecule, a PAH Oil Compound
The National Oceanic and Atmospheric Association has studied many details of seafood safety after an oil spill (Yender, R., J. Michel, and C. Lord. 2002. "Managing Seafood Safety after an Oil Spill". Seattle: Hazardous Materials Response Division, Office of Response and Restoration, National Oceanic and Atmospheric Administration. 72 pp. response.restoration.noaa.gov/book_shelf/963_seafood2.pdf, accessed May 13, 2010).
10 Facts of the Seafood Safety and Oils Spills Report are:
1. The chemistry and biology of crude oil spills is complex, but certain aspects are understandable and measurable.
2. Not all components of crude oil spills are equally harmful, some oil components are more harmful than others.
3. Dissolved oil fractions, dispersed oil droplets, or oil coatings can contaminate seafood and make the food unsafe for eating.
4. Aromatic compounds in the dissolved fraction of oil are more dangerous to humans potentially because they are more soluble in water and can contaminate sealife and seafoods more readily than many other components in oil.
5. About 99% of these aromatic hydrocarbons are benzene, toluene, ethyl benzene, xylene (all 4 are termed the BTEX group); other substitued benzenes, and the 2- to 3- ringed PAHs (fluorene, naphthalene, dibenzothiophene, anthracene and various substitued analogues of these compounds).
6. The distribution of aromatic compounds in the spilled oil is an indicator of the potential for contamination of seafood from water exposure.
7 seafood of all types needs to be monitored and sampled for potential content of carcinogenic compounds.
8. public news and information of seafood contamination needs to be broadly and widely disseminated by all media.
9. toxin- overloaded seafoods from oil-tainted fishing needs to be restricted from sale for human consumption.
10. tainted foods with certain levels of potential carcinogens may be marketed when based on acceptable risk assessment parameters.
10 Facts of the Seafood Safety and Oils Spills Report are:
1. The chemistry and biology of crude oil spills is complex, but certain aspects are understandable and measurable.
2. Not all components of crude oil spills are equally harmful, some oil components are more harmful than others.
3. Dissolved oil fractions, dispersed oil droplets, or oil coatings can contaminate seafood and make the food unsafe for eating.
4. Aromatic compounds in the dissolved fraction of oil are more dangerous to humans potentially because they are more soluble in water and can contaminate sealife and seafoods more readily than many other components in oil.
5. About 99% of these aromatic hydrocarbons are benzene, toluene, ethyl benzene, xylene (all 4 are termed the BTEX group); other substitued benzenes, and the 2- to 3- ringed PAHs (fluorene, naphthalene, dibenzothiophene, anthracene and various substitued analogues of these compounds).
6. The distribution of aromatic compounds in the spilled oil is an indicator of the potential for contamination of seafood from water exposure.
7 seafood of all types needs to be monitored and sampled for potential content of carcinogenic compounds.
8. public news and information of seafood contamination needs to be broadly and widely disseminated by all media.
9. toxin- overloaded seafoods from oil-tainted fishing needs to be restricted from sale for human consumption.
10. tainted foods with certain levels of potential carcinogens may be marketed when based on acceptable risk assessment parameters.
Toxicity, Carcinogenicity of Burned or Crude Oil Components – Polycyclic Aromatic Hydrocarbons (PAHs)
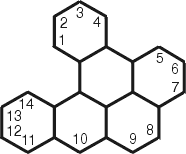
Dibenzopyrene
Common Polycyclic Aromatic Hydrocarbons (PAHs) of Burning are Potential Carcinogens
Burning oil, coal, tar, asphalt, and even food (charcoal grilling), may produce one or more of the PAH compounds listed here. This list of 15 chemicals represents compounds that are "reasonably anticipated" to be human carcinogens (first published (1981) in the Second Annual Report on Carcinogens, 1981).
To make this listing more understandable note that benzene rings are common, and all are categorized or fall into one of these four types: fluranthenes, acridines, pyrenes and chrysene.
Carcinogenicity of the compounds below was based on validated research done on experimental animals (IARC 1973, 1983, 1987). The cancers induced in these studies were of these types: papillomas, carcinomas, sarcomas and included tissues and organs of the skin, stomach, liver, bladder, and kidney. Carcinogenic compound names of each PAH is followed by the CAS Registry Number in parentheses.
benz[a]anthracene (56-55-3)
benzo[b]fluoranthene (205-99-2)
benzo[j]fluoranthene (205-82-3)
benzo[k]fluoranthene (207-08-9)
benzo[a]pyrene (50-32-8)
dibenz[a,h]acridine (226-36-8)
dibenz[a,j]acridine (224-42-0)
dibenz[a,h]anthracene (53-70-3)
7H-dibenzo[c,g]carbazole (194-59-2)
dibenzo[a,e]pyrene (192-65-4)
dibenzo[a,h]pyrene (189-64-0)
dibenzo[a,i]pyrene (189-55-9)
dibenzo[a,l]pyrene (191-30-0)
indeno[1,2,3-cd]pyrene (193-39-5)
5-methylchrysene (3697-24-3)
(Source: http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s150pah.pdf, accessed May 11, 2010)
See more important ideas and facts of crude oil spill control, cleanup and remediation procedures.
VISIT Our Science Super School Store
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.
Burning oil, coal, tar, asphalt, and even food (charcoal grilling), may produce one or more of the PAH compounds listed here. This list of 15 chemicals represents compounds that are "reasonably anticipated" to be human carcinogens (first published (1981) in the Second Annual Report on Carcinogens, 1981).
To make this listing more understandable note that benzene rings are common, and all are categorized or fall into one of these four types: fluranthenes, acridines, pyrenes and chrysene.
Carcinogenicity of the compounds below was based on validated research done on experimental animals (IARC 1973, 1983, 1987). The cancers induced in these studies were of these types: papillomas, carcinomas, sarcomas and included tissues and organs of the skin, stomach, liver, bladder, and kidney. Carcinogenic compound names of each PAH is followed by the CAS Registry Number in parentheses.
benz[a]anthracene (56-55-3)
benzo[b]fluoranthene (205-99-2)
benzo[j]fluoranthene (205-82-3)
benzo[k]fluoranthene (207-08-9)
benzo[a]pyrene (50-32-8)
dibenz[a,h]acridine (226-36-8)
dibenz[a,j]acridine (224-42-0)
dibenz[a,h]anthracene (53-70-3)
7H-dibenzo[c,g]carbazole (194-59-2)
dibenzo[a,e]pyrene (192-65-4)
dibenzo[a,h]pyrene (189-64-0)
dibenzo[a,i]pyrene (189-55-9)
dibenzo[a,l]pyrene (191-30-0)
indeno[1,2,3-cd]pyrene (193-39-5)
5-methylchrysene (3697-24-3)
(Source: http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s150pah.pdf, accessed May 11, 2010)
See more important ideas and facts of crude oil spill control, cleanup and remediation procedures.
VISIT Our Science Super School Store
All the Written Material within Site is Copyrighted 2010 and Owned by Dr. Donald Reinhardt, and this original material is protected legally by this copyright notice and by the Digital Millennium Act. None of this original material may be copied or reproduced without the expressed written consent of the author.
The author is a Freelance Science writer, and is available for specific assignments for those who are interested – by contacting adminstrator@sciencesuperchool.com. Other questions related to this teaching site should be directed to teacher@sciencesuperschool.com.